When a Match Burns Some Matter Is Destroyed
When a match burns some matter is destroyed. The match weighs less after burning.
3 7 Conservation Of Mass There Is No New Matter Chemistry Libretexts
Thats the one I was just talking about.

. This chemical reaction destroys matter. Let her see is definitely a law because it is it is the law of conservation of matter. What is the reason for your answer.
Matter is neither created nor destroyed. The mass of ash is less than the match it came from. So right there you can see that the mass-energy of the match is not conserved since it imports mass in the form of those oxygen molecules.
C The mass of ash is less than the match it came from. This is what you see when you light up a match and look extremely close at two thousand frames per second. What is the reason for why matter is not destroyed when a match burns.
Burning is defined as the rapid oxidation of a combustible material. This chemical reaction destroys matter. Earn 20 pts.
Fuel Any material that will release energy during a controlled chemical or nuclear reaction. The match weighs less after burning. When a match burns some matter is destroyed.
This means some molecules are destroyed while new ones are formed. Complete oxidation of the match stick results in an entirely different compound the ash while electrons are lost by the wood of the match. Energy is released when matter is destroyed Mass and energy are equivalent The law of conservation of energy must be modified.
The match weighs less after burning. Um this is most likely going to be an observation. The mass of the ash cannot be accurately determined.
Some of those combustion products however are gaseous and they escape the match. The atoms are not destroyed they are only rearranged. The European Chemistry Thematic Network expounds that in wood combustion a chemical reaction occurs.
Matter cannot be created or destroyed only change form. When someone lights a match they have started a small fire. The chemical reactions of the burning phosphorus and gelatin is.
This chemical reaction destroys matter. The atoms are not destroyed they are only rearranged. So it is a law and letter D.
Get started for FREE Continue. Ash is a low energy product and can easily get blown away therefore causing reduced mass New questions in Chemistry. In a burning log the initial mass of the wood and the ashes that are left behind after it burns are different which would seem to violate the law of conservation of mass.
Apr 8 2018. A This chemical reaction destroys matter. NO as the match burns is converts the chemical energy stored in the wood into heat fire and the resulting product is ash.
The mass of ash is less than the match it came from. What happens to the mass of a Match when it burns. When a match burns some matter is destroyed True or False.
This is well beyond the scope of a physical change. When wood burns oxygen and sugar molecules disappear and they are replaced by carbon dioxide and water molecules. When a match burns he is released.
So in other words mass cannot be created nor destroyed. Add your answer. Whats in the smoke.
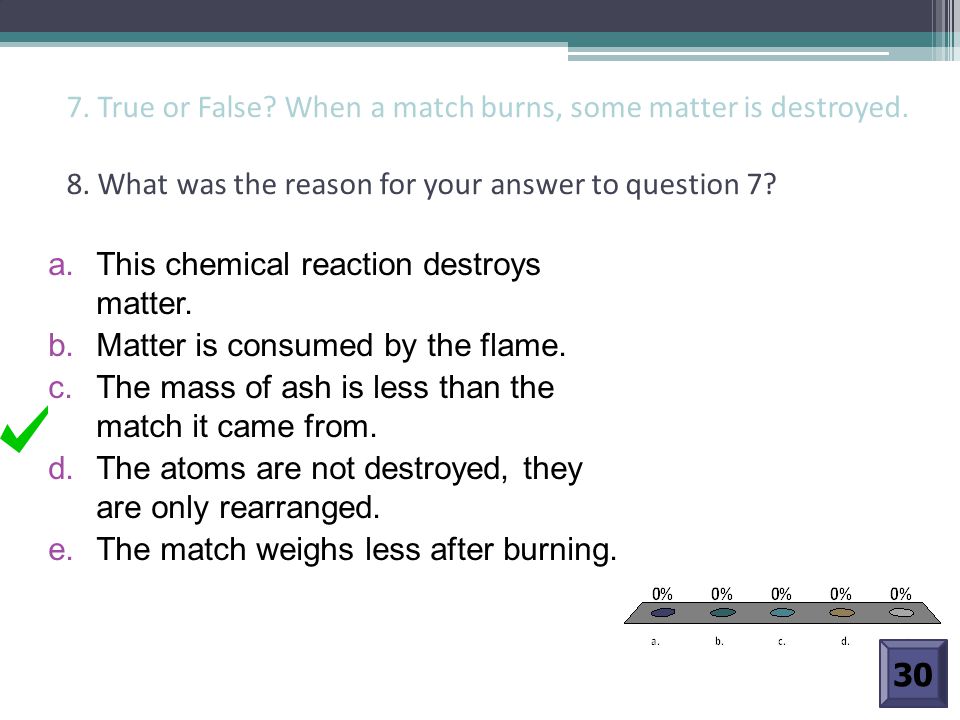
What is the reason for your answer to question 7. Matter is consumed by the flame. In a chemical reactiontwo or more substances called the reactants form different substances called products.
Matter is consumed by the flame. Dust to Dust Please do not try this without adult supervision We start with a match. The mass of ash is less than the match it came from.
The weight of the ashes after the burn is 10 kg. The atoms are not destroyed they are only rearranged. The atoms are not destroyed they are only rearranged.
Some of the matter is destroyed during the reaction. What is the reason for your answer to question 7. Who has discord bc I do if I want to talk to me tell ur thing for it.
A 300 kg tree burns to the ground leaving only ashes. CO2 and Water Vapor Heat smoke light. Lighting a match and letting is burn is an example of a chemical change.
Vinegar in baking soda. A burning match is a chemical change. Combustion is the process of burning.
When a match burn some matter is destroyed. So there is obvious mass loss as mass is transferred from the match to the environment. Moves in cycles from nonliving things to living things back to nonliving etc.
The law of conservation of mass states that for any closed system the mass of the system must remain constant. Fossil fuels coal natural gas and. Hasnt dissolved as much solute as is theoretically possible.
Fire The burning of some fuel creating a flame that releases light and heat. When a match burns some matter is destroyed. Element Each of more than one hundred substances that cannot be broken down into simpler substances.
This fire burns at two thousand flames per second. Molecules are not conserved in chemical reactions. Matter is consumed by the flame.
Chemical reactions cause chemicalchanges. B Matter is consumed by the flame.

Conservation Of Mass Ck 12 Foundation

0 Response to "When a Match Burns Some Matter Is Destroyed"
Post a Comment